Transient Gene Therapy For Microvascular Restoration
Foment Bio’s off-the-shelf gene constructs activate in vivo regenerative exosome production to renew microvasculature, resolve inflammation & restore tissue function.
Restoring tissue microvasculature is critical for clinically meaningful tissue remodeling and function recovery. Unfortunately, no currently available therapeutics are able to restore and maintain the microvasculature effectively. This is a distinctive feature of Foment therapy which has demonstrated microvasculature restoration across multiple animal models and clinical studies. Additionally, interactions with the FDA have confirmed that tissue vessel density is a valid biomarker, representing that multiple disease drivers (microvascular degeneration, inflammation, cell senescence) have been addressed, and is highly correlated with tissue function recovery.
The Importance of Restoring Microvasculature
Tissue Remodeling via Microvascular Restoration
Damage from aging & cardiometabolic dysfunction, at the tissue level, is driven by three interconnected biological processes: chronic inflammation, microvascular degeneration and cellular senescence. Together, these changes impair oxygen and nutrient delivery, sustain low-grade inflammatory signaling, and reduce the regenerative capacity of cells, ultimately leading to progressive tissue dysfunction. Systemically, we often experience and categorize this as aging, but when concentrated at the organ level it presents clinically as chronic disease.
Foment’s transient gene therapy constructs drive regenerative exosome release that directly addresses these mechanisms to remodel the tissue from within: restoring vascular density, resolving inflammation, and rescuing cells from senescence and death. This approach counters the biology of aging and cardiometabolic dysfunction and translates into measurable improvements in:
This has broad applications across age related diseases but unlike most longevity interventions, Foment’s therapy uniquely bridges aging biology and clinical medicine, enabling development through established regulatory pathways with defined indication, endpoints, and therapeutic benefit.
Heart Failure
Chronic Kidney Disease
Erectile Dysfunction
Wound Healing
Targeting the Root Drivers of Chronic Disease
Vasculogenesis
Inflammation Resolution
Cellular Rescue
Exosomes have been identified as key regulators of tissue remodeling in response to injury. These tiny bubble-like vesicles are released by cells into the microenvironment and play a critical role in intercellular communication, transporting bioactive molecules such as proteins and micro RNA, influencing recipient cell gene expression and behavior.
With this understanding, Foment Bio is developing non-viral, off-the-shelf, gene constructs that activate in vivo regenerative exosome production. The elevated exosome release drives microvasculature vessel growth, resolves inflammation and prevents cell death. This robust therapeutic approach is able to restore tissue function while addressing key markers of aging.
Our Approach: In Vivo Exosome Production
Foment’s plasmid gene therapy platform harnesses the body’s own cellular machinery to produce regenerative exosomes in vivo. Plasmids are small circular DNA molecules that deliver temporary genetic instructions to cells, enabling them to produce specific proteins without altering the cell’s genome.
By providing transient genetic instructions directly to tissue, this approach replaces the complex and costly ex vivo manufacturing required for cell and exosome-based therapies with a simple, efficient, self-limiting in vivo process. Additionally, unlike other genetic modalities, plasmid DNA offers a unique combination of inherent stability, low-cost, scalable manufacturing, and suitability for repeat dosing.
Foment’s transient gene therapy achieves the biological potency of high-dose exosome therapy in a stable, scalable, and inexpensive off-the-shelf vial.
The Plasmid Advantage
Translation
Foment Bio’s technology has been developed through a methodical, stepwise translational process from mechanism to clinical validation. Early in vitro studies demonstrated that our proprietary constructs drive significant increases in cell exosome production, confirming the intended mechanism of action. Subsequent in vivo animal studies established clear biological efficacy, with biomarker changes aligning with the observed functional improvements. These preclinical findings have since been translated into the clinic, where multiple FDA Phase 2 studies have shown biological activity and consistent, measurable benefits over placebo. This progression from mechanistic confirmation to clinical therapeutic benefit underscores the robustness and translational fidelity of Foment’s regenerative gene therapy platform.
IND-enabling studies utilizing a porcine model of heart failure were conducted to determine safety and therapeutic efficacy potential. In the studies, the treated pigs exhibited increased the microvascular vessel density in the cardiac tissue; 40-50% greater density than the control pigs. The improved vascularity correlated with tissue function improvements.
Preclinical Heart Failure Model
Biomarker Assessment
Functional Assessment
The potential of this approach has been translated into the clinic. To date, 203 patients have been treated with Foment Bio constructs with no drug related serious adverse events (SAEs). Efficacy signals have been observed across multiple indications.
In a Phase I study of dermal scarring, treatment arms reported meaningful improvement in scar cosmesis or appearance as assessed by both patients and physicians.
Clinical Translation
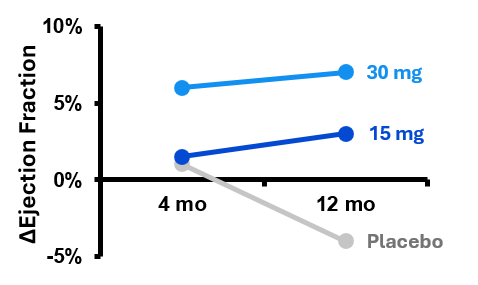
In a 93 patient, 16-site, double-blinded, Phase II heart failure study, the 30mg dose exhibited a significant ejection fraction improvement over placebo in a cohort of the most severe heart failure patients. Further, a clear dose response was observed; a strong indication of therapeutic biological activity.
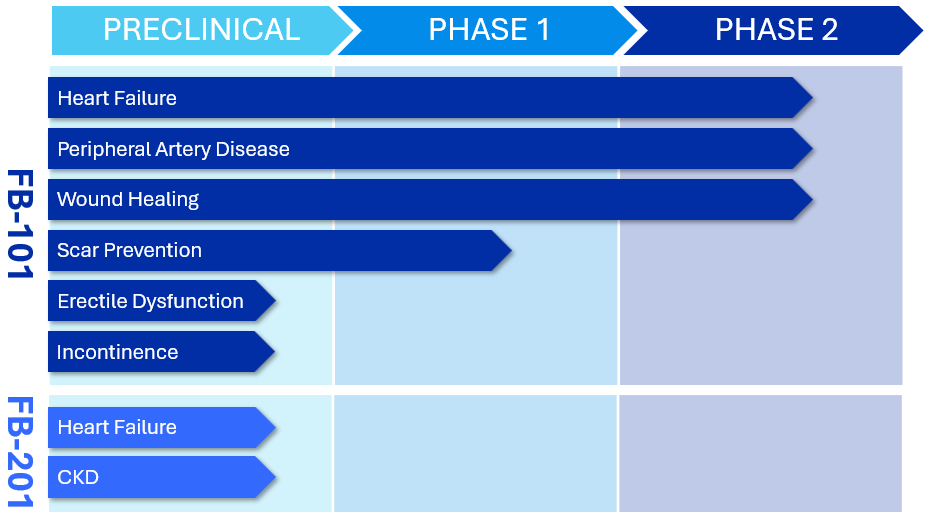
Product Pipeline
203 patients treated in FDA clinical studies
Strong Safety Profile: no drug related serious adverse events (SAEs)
Meet The Foment Bio Team